As CPRIT looks ahead to the next decade, we are guided by our three-part mission: investing in the research prowess of Texas institutions; creating and expanding the state’s life science ecosystem; and expediting innovation and enhancing the potential of breakthroughs in research, prevention, and cures.
Investing in the Research Prowess
of Texas Institutions
CPRIT Funds 67 Core Facilities at 22 Institutions
Breakthrough discoveries create promising cancer-fighting opportunities, but scientists need specialized equipment and expertise to turn their discoveries into treatments and cures. CPRIT’s Core Facility Support Award grants make it possible for Texas institutions to build state-of-the-art facilities, acquire cutting-edge equipment, and recruit leading experts. The network of 67 CPRIT-funded core research facilities across 22 Texas institutions fosters partnerships and connects scientific disciplines and departments working on a wide variety of cancer-related projects.
CPRIT’s Core Facilities bring together sophisticated technologies and innovative projects, bolstering Texas’ life science ecosystem. By assuring access to specialized equipment and expertise, CPRIT provides Texas researchers efficient, collaborative, cost-effective means for advancing innovations in cancer medicine. With CPRIT’s support, Texas scientists are conducting first-of-its-kind cancer studies, creating the most expansive cancer databanks in the world, and collaborating with the brightest minds in cancer research and prevention.
Featured Core Facilities

The GCC Combinatorial Drug Discovery Program
The Gulf Coast Consortia Combinatorial Drug Discovery Program (CDDP) is a multi-institutional core facility based at the Texas A&M Institute of Biosciences and Technology. The CDDP provides cancer researchers across Texas with access to state-of-the-art equipment, drug libraries, and expertise to support cancer-related drug discovery research.
One focus for the CDDP is helping researchers find new ways to use existing drugs to treat different types of cancer. The advantage of redirecting “old" drugs to "new" uses - known as drug repurposing - is that a great deal is already known about the properties of established drugs, making it much cheaper and faster to get them into the clinic than if they were brand new drugs. The CDDP also specializes in phenotypic screening studies using high-throughput, high content, flow cytometry, and metabolic imaging screening platforms.
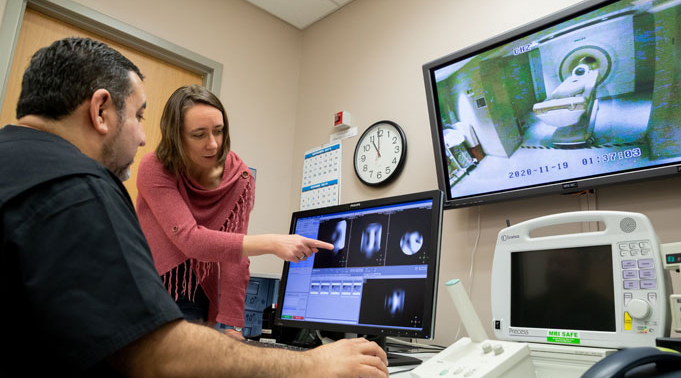
The North Texas Multimodal Small Animal Imaging Core Facility
As a world-class pre-clinical imaging facility, the North Texas Multimodal Small Animal Imaging Core provides invaluable opportunities for Texas scientists to advance the study of human disease. Located in the Harold C. Simmons Comprehensive Cancer Center at The University of Texas Southwestern Medical Center, this CPRIT-funded core facility, under the direction of CPRIT Scholar Dr. Anke Henning, supports the research of more than 100 different scientists from institutions across North Texas.
With the CPRIT-funded ultra-high-resolution imaging equipment, researchers are developing new diagnostic imaging techniques to improve the way scientists classify and treat cancer and other diseases. The spatial imaging equipment is precise enough to pinpoint the individual atom clusters that play a key role in treatment resistance, opening the door for researchers to find new ways to detect, treat, and cure various cancers and diseases.
The CPRIT RNA Therapeutics Core
Established in 2015 and expanded in 2020, the CPRIT RNA Therapeutics Core at Methodist Hospital Research Institute, under the direction of Dr. John Cooke, is a valuable state-of-the-art resource that helps 50 Texas R&D groups from research institutions and biotech firms translate their ideas and innovations into therapies.
RNA therapeutics is an exciting new class of drugs to defeat cancer. RNA is a form of biological software that can instruct a cell to make proteins that can protect against cancer. The CPRIT RNA Therapeutics Core provides unique services in development, manufacturing, quality control, as well as animal and human testing of RNA drugs. In the video, Dr. Cooke and his colleagues explain the promise of RNA therapeutics and the collaborative work enabled by the state-of-the art facility.
Creating and Expanding
Texas Life Science Infrastructure
A Region-by-Region View of Life Science Ecosystem
Through a decade of steady investment, CPRIT has built an indispensable network of institutions, enterprises, researchers, and care providers dedicated to the prevention and cure of cancer. Enhancing Texas’ life science ecosystem requires resources such as core facilities, advanced manufacturing tools, state-of-the-art computational modeling technologies, accelerator hubs, and other critical assets. The most effective way to reduce the burdens of cancer is through evidence-based prevention projects, including education, vaccination, and early screening and treatment services, which focus on underinsured and medically underserved populations throughout the state.
CPRIT's investments bring the latest technologies and the brightest researchers to Texas and give Texans more opportunities to access cancer prevention services in underserved regions. By integrating unmatched biological, medical, chemical, and computational expertise with advanced technology, CPRIT has primed Texas’ workforce for new company development. Click on each region to see some examples of CPRIT’s recent investments in Texas’ life science ecosystem.

Accelerating Innovation in Preventing and Treating Cancer
Advances in technology over the past decade open new doors for patient-specific treatment. But these groundbreaking cancer discoveries made in university laboratories across Texas are most valuable when scientists and drug developers can translate those findings into new therapies available to cancer patients. An important part of CPRIT’s mission to develop innovative cancer treatments is supporting clinical study research conducted by Texas-based companies and research institutions.
Despite progress made in treating cancer, preventing cancer from developing and detecting it early if it does are still the best options to reduce the physical, economic, and emotional burden of cancer for all Texans. Innovations in science and technology provide new avenues for cancer prevention and detection.
Clinical Studies – Roadmap to Innovation
CPRIT’s investments in clinical research and development generate long-lasting gains for the state’s life-science ecosystem. New cancer drugs, medical devices, and technologies must undergo clinical safety and efficacy tests to receive FDA approval. This testing, called the clinical trial process, is vital to the development of life-saving treatments, such as precision therapy drugs and other groundbreaking cancer therapies.
Many factors, including genetics, race, sex, age, and socioeconomic issues, influence how a patient responds to treatment. As important as it is to fund clinical trials of potential breakthrough cancer treatments, it is also critical to increase the number of people participating in clinical trials. Currently, less than 5% of patients with cancer participate in clinical trials.
The Clinical Trial Network Award and the Texas Clinical Trial Participation Program Award grants support clinical trials in underserved regions of Texas and reduce financial barriers to patient participation in clinical trials. In 2021 CPRIT awarded its first grants intended to expand access to clinical trials in Texas.
CPRIT grants invest in the development of innovative treatments and interventions at various phases from preclinical development to market launch. Below are some examples of recent achievements reached by CPRIT grantees during key phases of the clinical trial process.
Preclinical Development (Pilot Phase)
Deciding whether a drug is ready for clinical trials involves extensive preclinical studies using in vitro (test tube or cell culture) and in vivo (animal) experiments that yield preliminary efficacy, toxicity, pharmacokinetic and safety information. Scientists may also perform in silico profiling using computer models of the drug–target interactions.
Phase 1 Clinical Development
Phase 1 trials are the first tests of a drug in humans, usually with a small number of patients to assess the safety and tolerability of a drug.
Phase 2 Clinical Development
Phase 2 trials involve larger groups of patients to assess the effectiveness of the drug and to continue the Phase 1 safety assessments. Phase 2 clinical studies help to establish therapeutic doses for the large-scale Phase 3 studies.
Innovation in Cancer Prevention – Taking Texas Tobacco Free
Tobacco use is the foremost preventable cause of death and disease in the country. Despite recent declines in smoking rates, more than 480,000 Americans die from first- and second-hand cigarette smoke each year. Contributing to more than 30% of all cancer deaths nationwide, scientists link tobacco use to at least 17 types of cancer—including the oral, esophageal, and pancreatic cancers associated with smokeless tobacco.
Texas’ annual rate of tobacco-related mortality is higher than the national average, which is why effective prevention programs are vital to stopping premature death and disease in the state. Researchers also know that compared to the general population, individuals with mental health conditions, substance abuse disorders, and vulnerable housing arrangements use tobacco at significantly higher rates and have a higher risk of developing lung and other cancers.
The timeline below shows the evolution of the Taking Texas Tobacco Free (TTTF) initiative, beginning with its launch in 2013 by The University of Texas M.D. Anderson Cancer Center, Rice University, the University of Houston, and Austin Travis County Integral Care with CPRIT funding. (PP130032). Led by program director Dr. Lorraine Reitzel and her team at the University of Houston, TTTF has expanded to 39 geographic service areas in the state. To date, TTTF’s tobacco cessation resources have helped 23 community mental health clinics (PP160081), 18 substance use treatment centers (PP170070), and nine community agencies that serve homeless and vulnerably housed individuals (PP210026). These resources include comprehensive educational tools, ongoing training opportunities, and adaptive curriculum directives designed to gradually reduce tobacco use among state employees and the Texans they serve (PP200051).
Overall Impact of Taking Texas Tobacco Free
TREATING TOBACCO
CONDUCTED
AGENCIES
MATERIAL REACH